
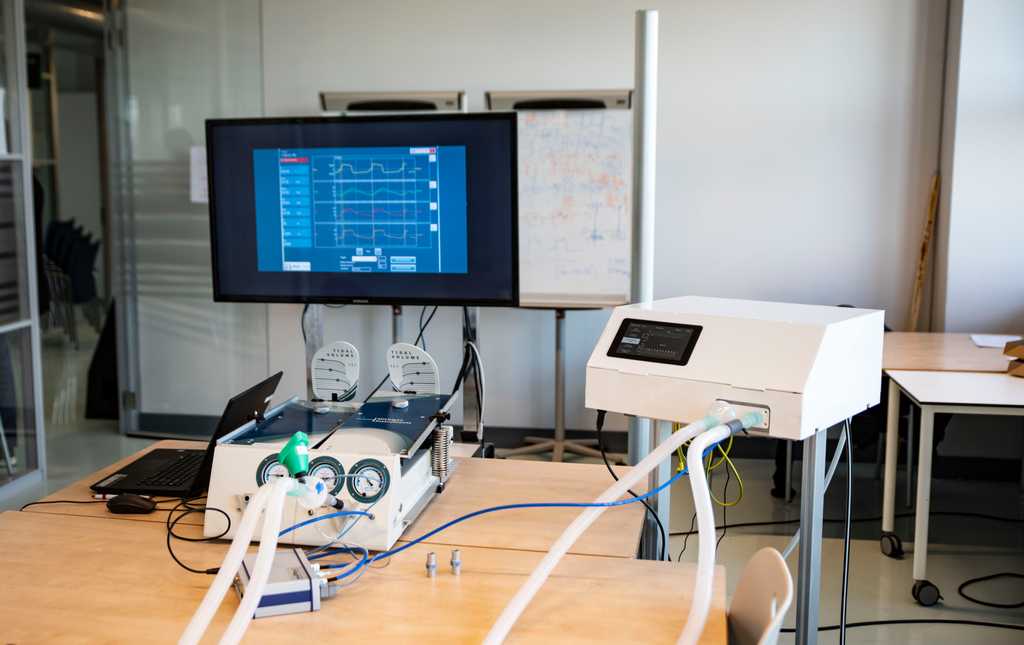
Our first functional prototype is a fact: the AIRone. Last week the AIRone has been a subject to various tests. Using a mechanical phantom of the lungs we were able to do the functional tests that determine if the prototype meets our requirements. The documents are submitted to the Ministry of Health, Welfare and Sports, as the start of the clinical tests are required to be authorized by them. Clinical approval is obliged before our ventilator can be used during the coronavirus pandemic. If clinical approval is granted, the Ministry of Health, Welfare and Sports can instruct production of our ventilators.
We have started a cooperation with an assembly facility in the region to be able to start the production as soon as possible when we receive clinical approval. Furthermore, we have close contact with suppliers of the different parts of the AIRone. When the Ministry of VWS instructs us to start production of our ventilators, we can start immediately with our partners. Our ambition is to be able to produce 500 ventilators in total by producing 100 ventilators a week. If the demand for ventilators increases, we could possibly decide to scale up. We have close contact with the Dutch Intensive Care Society and the Dutch National Consortium of Clinical Supplies to stay up-to-date on the demand for ventilators in the Netherlands. For now, though, our main focus is getting clinical approval.
When clinical approval is granted and the production has started, OperationAIR will continue operating. The team will focus on implementation, service and further improvement. A training plan regarding our ventilator is set up for medical professionals in the hospital. Furthermore, a team is assembled that will be ready to provide assistance when the ventilator is implemented in a hospital. The clinically approved design will be publicly available on our website, so other countries can make use of it as well.


View original article here.

