Technological advancements continue to shape the way professionals acquire critical skills in medical education and research.
One such innovation that stands out is the integration of respiratory training simulators, specifically lung simulators, into the educational toolkit of medical professionals, ventilator manufacturers, and educators.
Among the pioneers in this field is Michigan Instruments, offering state-of-the-art lung simulators paired with PneuView Software.
What Sets Our Lung Simulators and PneuView Software Apart?
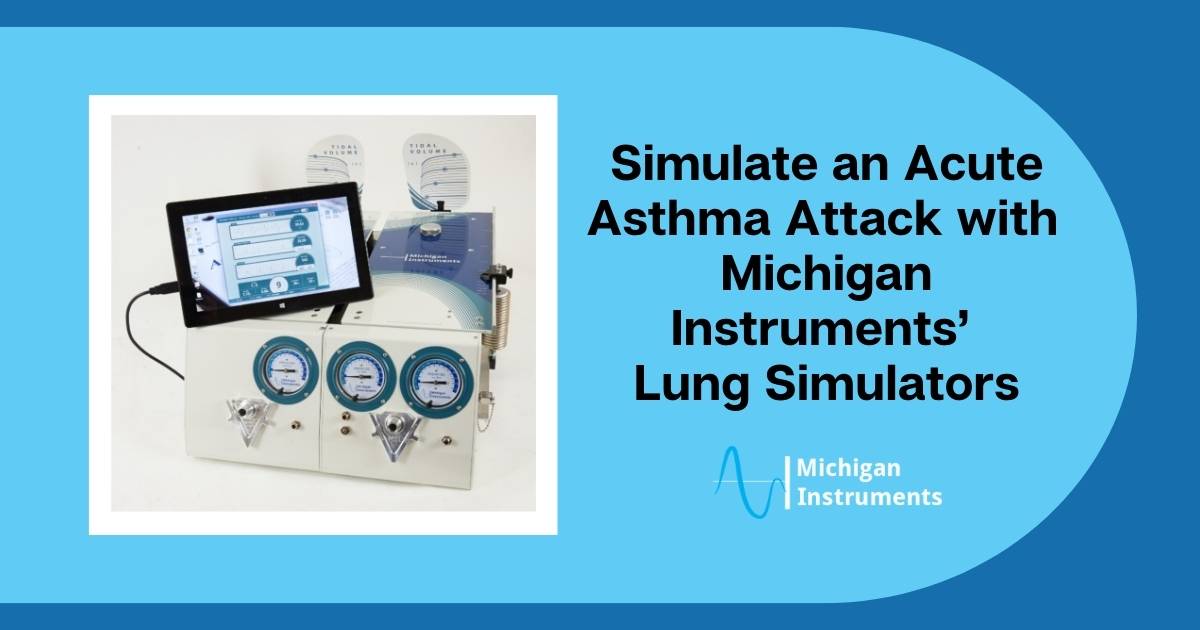
Michigan Instruments takes pride in providing lung simulators that are fully to scale, offering realistic residual lung volumes and capacities. The range of settings for compliance and resistance on TTL® and PneuView® systems surpasses most other available simulators. What makes these devices truly exceptional is their ability to move and “feel” like real lungs when ventilated.
Unlike basic test lungs that perform only a handful of simulations and lack full scalability, Michigan Instruments’ lung simulators offer a superior level of realism.
This realism is not just a feature; it is a crucial aspect that enhances the utility of these devices for hands-on training, product testing, and research and development.
Unparalleled Realism for Lifesaving Education
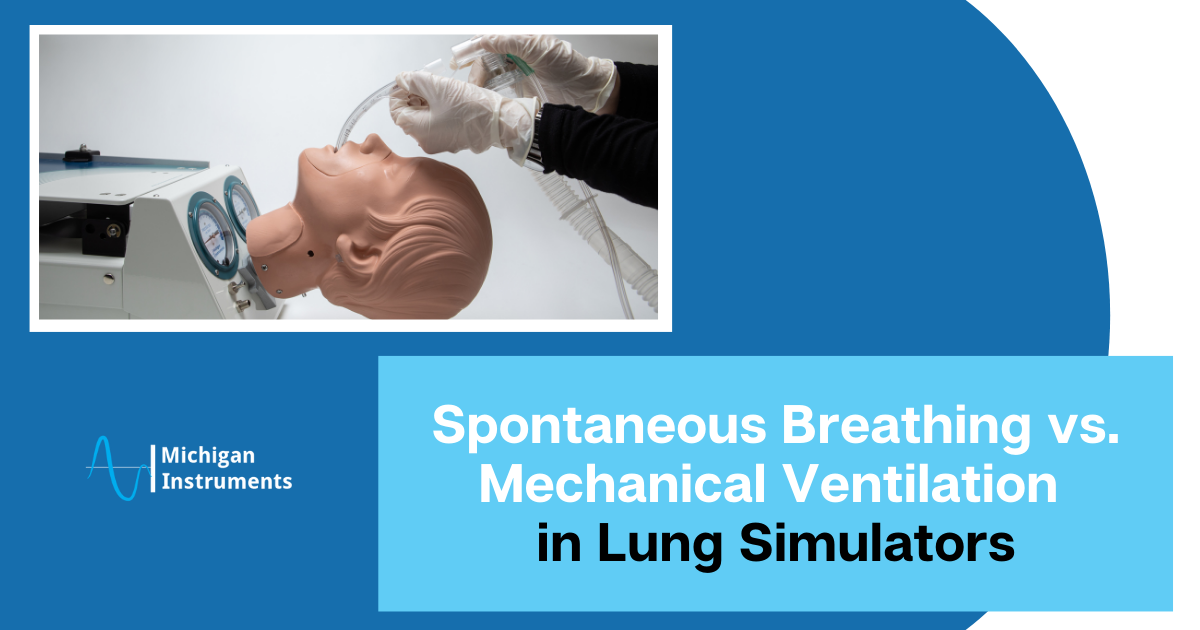
The combination of Michigan Instruments’ Lung Simulators and the PneuView Software creates a more realistic environment for medical professionals and students.
These devices go beyond basic simulations, providing an experience that more closely replicates the complexities of the human pulmonary system.
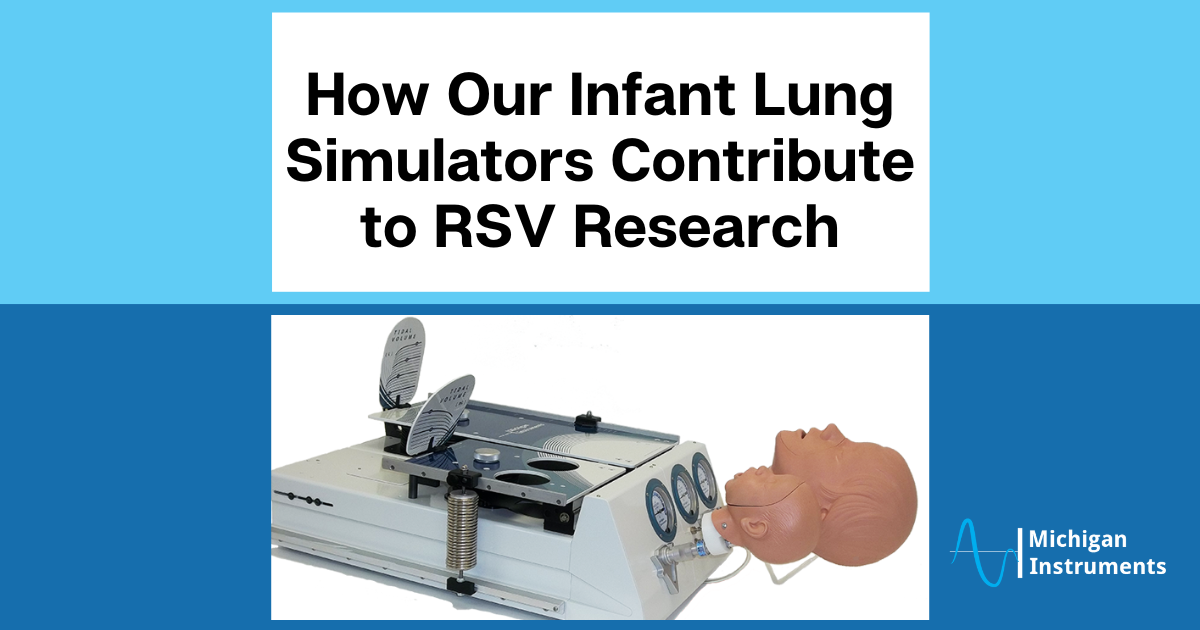
The realistic representation of adult and infant lung conditions allows for a diverse range of simulations, enhancing understanding and clinical skills development.
Why Choose Michigan Instruments?
There are several reasons to choose Michigan Instruments, just as facilities around the world have.
Pioneer in Respiratory Care and Emergency Medical Industries
Michigan Instruments has established itself as a pioneer in the respiratory care and emergency medical industries.
Our lung simulators and automated CPR devices have been recognized as breakthrough innovations in the medical profession.
Gold Standard of Respiratory Simulation
With thousands of users worldwide, Michigan Instruments’ lung simulators are considered the gold standard of respiratory simulation.
The devices have proven their efficacy in various applications, from medical education to testing to research and development.
Applications Across Industries
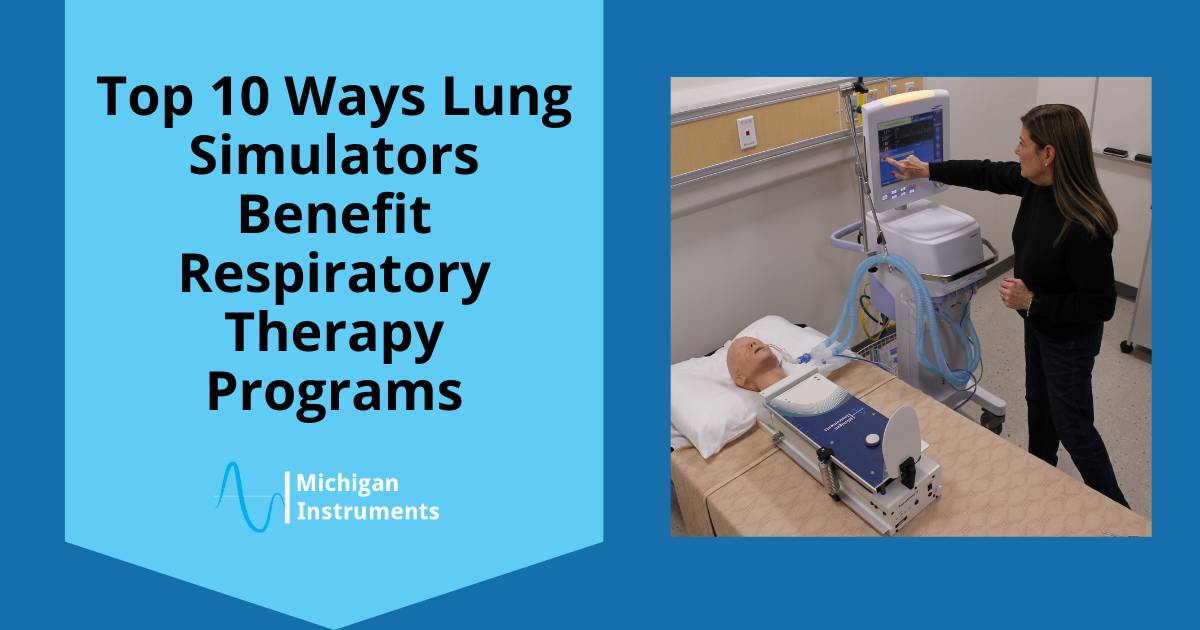
Classroom Simulation: Michigan Instruments’ TTL and PneuView Lung Simulators facilitate “Aha Moments!” in classrooms, offering hands-on experiences that enhance understanding and develop valuable clinical skills in students.
Research and Development: The lung simulators play a crucial role in the design, engineering, evaluation, and manufacturing of respiratory devices. They contribute to making these devices more user-friendly and effective.
Testing: Evaluate the performance of respiratory devices, even in the face of changing pulmonary dynamics. PneuView Systems enable the collection and display of ventilation data through advanced software.
Request a Quote: Join the Ranks of Professionals Choosing Excellence
Discover why thousands of educators, researchers, manufacturers, and quality assurance professionals worldwide trust Michigan Instruments’ lung simulation devices.
Experience high-quality lung simulators that you can touch, see, and modify to meet the evolving demands of respiratory care and medical education.
Michigan Instruments is at the forefront of transforming respiratory training in the digital age, providing cutting-edge solutions that bridge the gap between theory and practice in the lifesaving field of respiratory care.