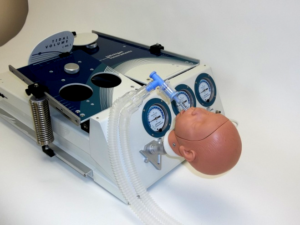
Grand Rapids, MI – Grand Valley State University students will soon have the opportunity to simulate the proper management of life-like respiratory ailments using the latest in training and test lung devices. Grand Rapids-based, Michigan Instruments Inc. developers of the world-renowned “Michigan Lung” plans to donate two TTL respiratory simulation units to the program, with a value of approximately $25,000. Grand Valley and Muskegon Community Colleges are collaborating to offer Muskegon’s Respiratory Therapy education for GVSU students. Nursing and Physician Assistant students at GVSU will also benefit from the simulation units.
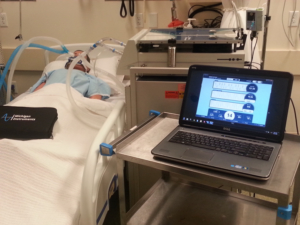
These sophisticated devices provide students with real-time data, measurements, and response that simulate those of a real respiratory patient. With this information, students learn how to properly ventilate and manage a variety of respiratory conditions.
“It is our privilege to provide the latest advancements in training and test lung products to a local program like Grand Valley State University’s,” stated Joe Baldwin, President of Michigan Instruments, Inc. “Our ‘Test Lungs’ are known and recognized worldwide and we are fortunate to work with a program like GVSU’s to ensure students in our community are able to receive the absolute best training possible right here in West Michigan.”
Michigan Instruments Inc, partnered with local software design and development firm, Atomic Object, to architect and develop cutting-edge software called “PneuView 3” — their latest training and test lung software application which calculates and displays, in real time, numerous respiratory parameters and waveforms. Software improvements combined with intricate design modifications to the Michigan Lung, provide users with even greater simulation capabilities.
The Michigan Lung is regarded as the most versatile, reliable training and test lungs on the market, and its latest multifaceted, fully to-scale mechanical design and software upgrades allow for simulation of hundreds of patient scenarios.
About Michigan Instruments
MII has designed and manufactured specialized medical equipment related to the fields of cardiovascular medicine, mechanical CPR, and respiratory therapy for over forty years. The company has built a reputation for medical device products of exceptional quality, which has earned the respect of thousands of customers, associates, and medical professionals throughout the world.
MII complies with Food and Drug Administration (FDA) and International Organization of Standardization (ISO) regulations for Good Manufacturing Practices. Both the FDA and ISO systems require continuous control over all activities that assure the quality of Michigan Instruments products and services. MII has built a strong foundation for growth based on the dedication of its’ staff, a close relationship For more information, contact us today.